Background: Primary and secondary [post-essential thrombocythemia (ET) or polycythemia vera (PV)] Myelofibrosis (MF) are clonal hematopoietic neoplasms marked by constitutive JAK-STAT activation, bone marrow fibrosis, cytopenias, and constitutional symptom burden. Allogeneic hematopoietic cell transplantation (HCT) is the only curative option in this disease. Median overall survival (OS) after JAK inhibitor (JAKi) discontinuation is poor, ranging from 6-24 months (Harrision et al. Ann Hematol. 2020). Haploidentical (Haplo)-HCT with post-transplant cyclophosphamide (PTCy) has improved donor availability, but data on post-HCT outcomes in MF remain scarce. Herein, we describe clinical outcomes of patients (pts) with MF who underwent haplo-HCT with PTCy.
Methods: We conducted a multi-institutional study where we retrospectively reviewed charts to obtain pt, disease, and treatment characteristics of MF pts who underwent haplo-HCT from 2000 to 2019. Graft-versus-host-disease (GVHD), relapse, and non-relapse mortality (NRM) were described as cumulative incidences. OS and relapse free survival (RFS) were estimated using the Kaplan-Meier method. Univariate analyses were conducted with Cox or Fine and Gray regression.
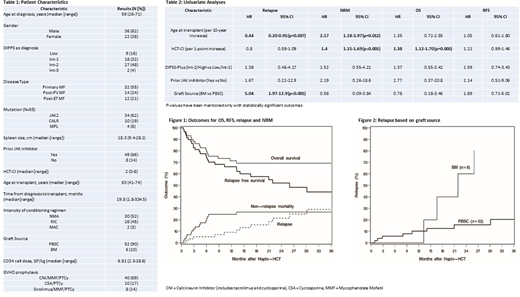
Results: Fifty-eight adult pts from 11 centers underwent haplo-HCT and were included in the analysis. Pt, disease, and HCT characteristics are listed in Table 1. Thirty-four (59%) pts were over 60 years of age at the time of HCT. Of the 53 pts in whom driver mutation data was available, JAK2 was reported in 34 (64%), CALR in 10 (19%), MPL in 4 (8%), and 5 (9%) were triple-negative. Twenty-two (38%) pts had HCT-CI ≥ 3. Median CD34+ cell dose was 6.81 (range: 2.3-28.6) x 106/kg. All pts received PTCy as a part of GVHD prophylaxis regimen. Median follow-up in this study was 28 (range: 3.3-75.7) months.
Neutrophil and platelet engraftment was reported in 53 (91%) and 47 (81%) pts at a median time of 20 (range: 14-70) and 31 (range: 15-225) days, respectively. Five pts (9%) had graft failure. The cumulative incidences of all-grade acute and chronic GVHD were in 44% (95% CI: 31-56%) at 6 months and 31% (95% CI: 18-44%) at 2 years. Grade 3-4 acute GVHD was seen in 5 (9%) pts. Post-HCT relapse/disease persistence occurred in 12 (21%) pts with a median of 416 (range: 28-917) days. The 2-year estimates of OS, RFS, relapse and NRM were 69% (95% CI: 55-80%), 52% (95% CI: 37-65%), 21% (95% CI: 11-34%) and 27% (95% CI: 16-39%) respectively (Fig 1).
On univariate analyses (Table 2), older age (HR 2.17, P=.012) and a higher HCT-CI (HR 1.4, P<.001) was associated with higher NRM. Higher HCT-CI was also associated with higher all-cause mortality (HR 1.38, P=.003). Bone marrow as a graft source was associated with a higher risk of relapse (HR 5.04, P<0.001, Fig 2), but did not significantly affect OS (HR 0.78, 95% CI: 0.18-3.46), RFS (HR 1.89, 95% CI: 0.71-5.02) or NRM (HR 0.58, 95% CI: 0.09-3.84). JAKi use prior to HCT, spleen size or type of driver mutation did not significantly affect outcomes.
Conclusions: Based on this study, we conclude that haplo-HCT with PTCy is a valid option in pts with MF. The graft failure rates appear to be similar to those reported with sibling and unrelated donors. Older age, higher HCT-CI and bone marrow as graft source were associated with inferior outcomes. JAKi use prior to HCT or type of driver mutation did not significantly affect outcomes.
Grunwald:Trovagene: Consultancy; Daiichi Sankyo: Consultancy; Merck: Consultancy; Agios: Consultancy; Janssen: Research Funding; Cardinal Health: Consultancy; Incyte: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; Forma Therapeutics: Research Funding; Amgen: Consultancy; Celgene: Consultancy; Incyte: Consultancy, Research Funding; Celgene: Consultancy; Celgene: Consultancy; Pfizer: Consultancy; Incyte: Consultancy, Research Funding; Merck: Research Funding; Astellas: Consultancy; Genentech/Roche: Research Funding; Premier: Consultancy; Premier: Consultancy; Astellas: Consultancy; Premier: Consultancy; Daiichi Sankyo: Consultancy; Agios: Consultancy; Abbvie: Consultancy; Merck: Consultancy; Pfizer: Consultancy; Trovagene: Consultancy; Trovagene: Consultancy; Abbvie: Consultancy; Abbvie: Consultancy; Agios: Consultancy; Forma Therapeutics: Research Funding; Forma Therapeutics: Research Funding; Astellas: Consultancy; Amgen: Consultancy; Amgen: Consultancy; Merck: Consultancy; Cardinal Health: Consultancy; Pfizer: Consultancy; Cardinal Health: Consultancy; Janssen: Research Funding; Genentech/Roche: Research Funding; Genentech/Roche: Research Funding. Dholaria:J&J: Research Funding; Takeda: Research Funding; Angiocrine: Research Funding; bms: Research Funding; Poseida: Research Funding. Abedin:Jazz Pharmaceuticals: Honoraria; Agios: Honoraria; Helsinn Healthcare: Honoraria; Pfizer: Research Funding; Helsinn Healthcare: Research Funding; Actinium Pharmaceuticals: Research Funding. Gupta:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy; Incyte: Honoraria, Research Funding; Bristol MyersSquibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees. DeZern:MEI: Consultancy; Celgene: Consultancy, Honoraria; Astex: Research Funding; Abbvie: Consultancy. Gerds:Sierra Oncology: Research Funding; Pfizer: Research Funding; Celgene: Consultancy, Research Funding; CTI Biopharma: Consultancy, Research Funding; AstraZeneca/MedImmune: Consultancy; Incyte Corporation: Consultancy, Research Funding; Apexx Oncology: Consultancy; Roche/Genentech: Research Funding; Imago Biosciences: Research Funding; Gilead Sciences: Research Funding. Jain:Bristol Myer Squibb: Other: for advisory board participation; Takeda: Consultancy, Honoraria; CareDx: Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal